views
The union ministry of health and family welfare offered relaxation to the rules about medical device registration. It is a great relief for the medical device industry, especially for micro, small and medium scale enterprises. It will grant a longer period for complying with ISO 13485 certification, which is needed for getting registration. It came at a time when the maximum manufacturer of medical devices reported that they couldn't do registration within September 30, when the time for voluntary registration ended because of all difficulties in obtaining ISO certification in the middle of the covid-19 pandemic and other disruption.

As per the draft amendment rule by the Ministry, there was an inclusion of provisions and explanations under rule 19B. It has been stated that proof of compliance based on ISO 13485 standard recognized by the National Accreditation Board for certification bodies or global accreditation forum concerning medical devices must be uploaded for registration on the online system for medical devices as per Central Drugs Standard Control Organization.
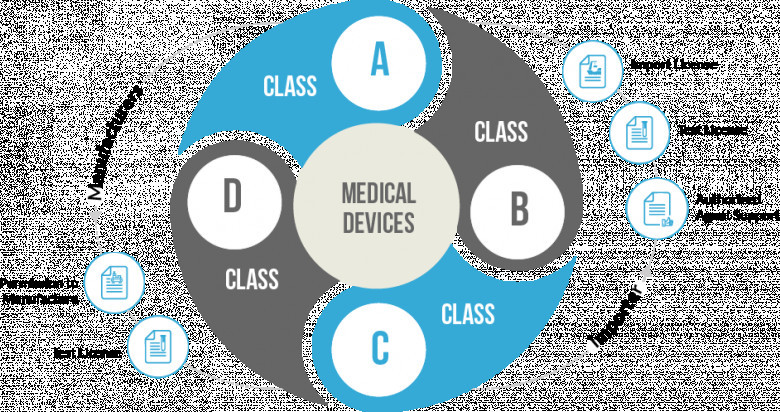
As per the draft amendment rule, when an applicant submits on or before November 30, 2021, an undertaking that the applicant must obtain ISO 13485 certificate on or before May 31, 2022. This must remain valid until May 31, 2022, or the date the applicant should obtain the ISO certificate, whatever is earlier. The draft also says that to remove doubts, such as in case the ISO 13485 certificate is not obtained before May 31, 2022, the provisional registration will be cancelled for every purpose without notice. In addition, as per Rule 19C, rather than the condition, the company must mention the registration number on the medical device label. This has been amended by substituting a condition that the manufacturer might be desired to give the registration number or the provisional registration number up to May 31, 2022; after the date, it will be compulsory for all registered holders to give the registration number in the label.
The draft also amended rule 19D sub-rule two items iii about medical device registration India by the importers. A proviso and explanation of the importer's effect should also submit an undertaking before November 30, 2021, and the company must obtain ISO 13485 certificate before May 31, 2022. A provisional registration number is generated that will remain valid till May 31, 2022. This number remains valid for every purpose. The relaxation has also been extended to mention registration numbers on the product label.
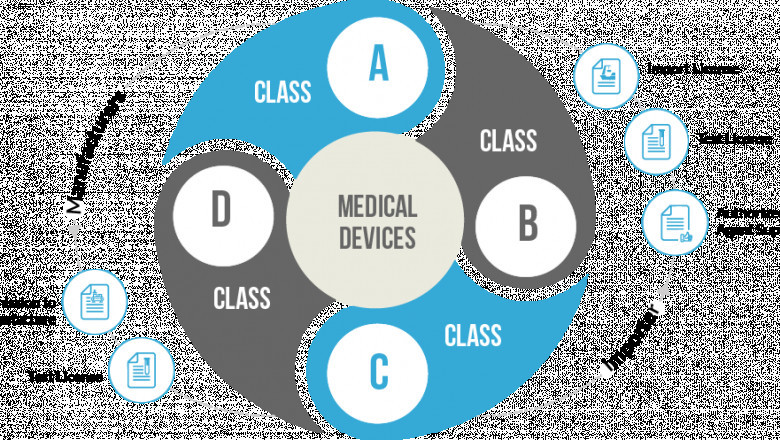
According to the Ministry of health and family welfare, medical devices fall under the voluntary registration scheme from April 1, 2020, until the end of September 2021. From October 1, 2021, all class A and B medical devices will come under the compulsory registration scheme until September 2022. Medical devices under class C and D will come under the compulsory registration scheme until September 2023.












